Pariente Delgado


HealthChain, is a project funded through the I3 Instrument that brings together the public and private sectors in the regional ecosystems to develop demand-driven digital health solutions through co-creation between Healthcare Organisations and IT companies.
Each participant region brings together the same structure of partners and roles:

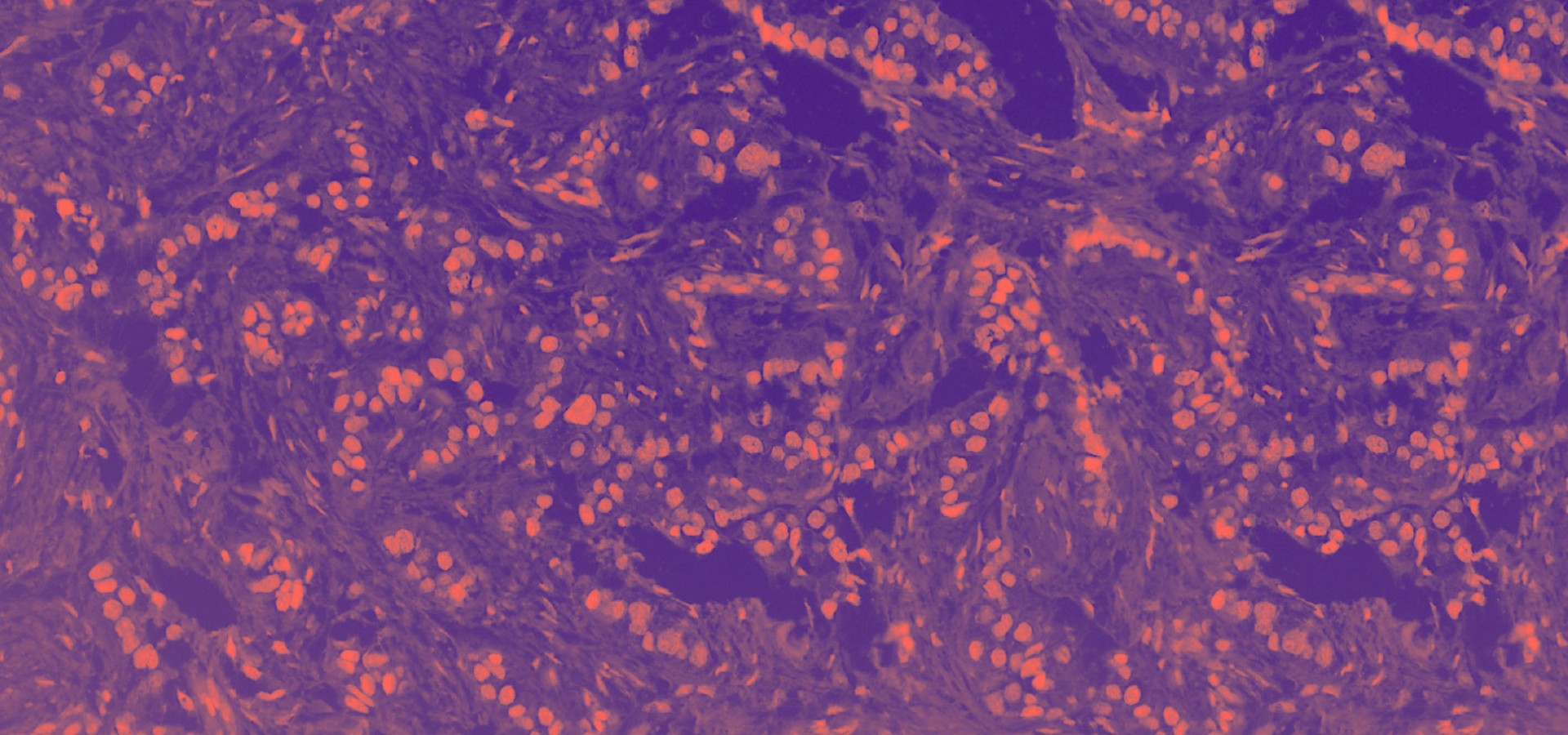
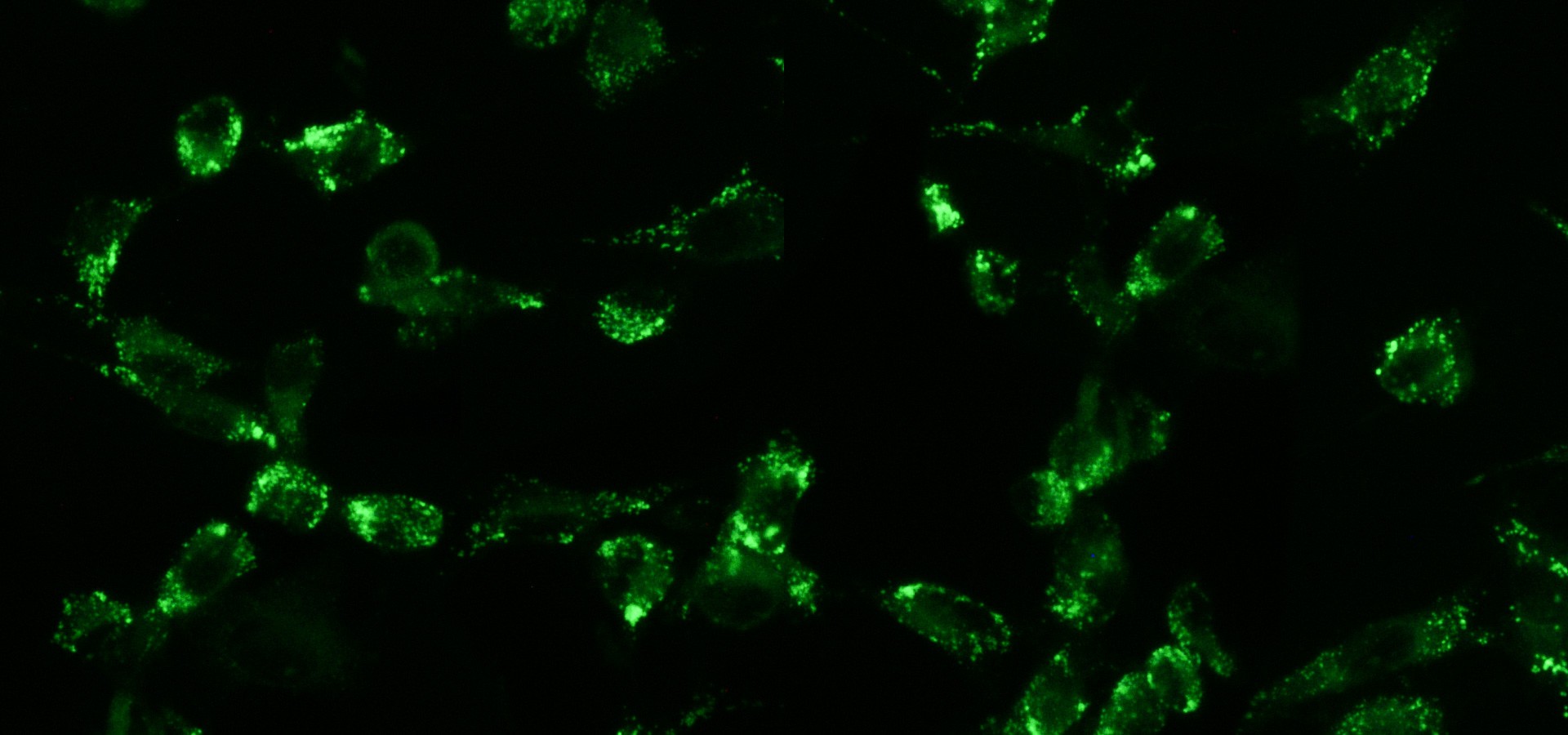
The Translational Medical Oncology Unit focuses on developing projects that facilitate the transition to precision medicine in the field of medical oncology in Navarra. To do this, we integrate translational research, understood as the link between the clinic and the laboratory, and basic research, understood as the study of the biology that characterises carcinogenesis and its progression. Our lines focus on the study of genetic alterations in both tumour biopsy and liquid biopsy, with the aim of identifying molecular biomarkers that facilitate the management of the disease. In addition, we study the immune component and its interaction with tumour cells, to characterise key cell populations in the development of new strategies in immunotherapy.
Lines of research:
Navarrabiomed-Centro de Investigación Biomédica
Hospital Universitario de Navarra, edificio de investigación.
C/ Irunlarrea 3. 31008 Pamplona, Navarra. España

Proyecto financiado por la convocatoria de Proyectos Estratégicos AECC 2022.


SISAQOL-IMI (945042 - Setting International Standards in Analysing Patient-Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials – IMI) is an international multidisciplinary consortium, co-led by the European Organization for Research and Treatment of Cancer (EORTC) and Boehringer Ingelheim (BI). The consortium has been convened to generate recommendations to standardize the use, analysis, and interpretation of patient reported outcome (PRO) data in cancer clinical trials.
SISAQOL-IMI will establish guidance on how to use patient-reported outcomes in cancer clinical trials so that they can be used in a methodologically sound way, analysed in a statistically adequate manner, and intelligibly presented to ensure a high study quality and a better comparability of results across clinical trials.
In june 2023, The Lancet - Oncology Magazine publishes the article: Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials-Innovative Medicines Initiative (SISAQOL-IMI): stakeholder views, objectives, and procedures.



Proyecto financiado por la convocatoria correspondiente al año 2022 de concesión de subvenciones de la Acción Estratégica en Salud 2021-2023, en el marco del Plan de Recuperación, Transformación y Resiliencia. Financiado por Fondos Next Generation.
FUNDING ORGANISATION:

Contrato financiado por la convocatoria de Ayudas para la contratación de personal investigador. Programa MRR Investigo 2022.
financiado por la Unión Europea – Next Generation EU




Proyecto de investigación financiado por el Departamento de Salud del Gobierno de Navarra a través de su convocatoria "Proyectos de Investigación en Ciencias de la Salud 2022"


Ayuda financiada por la convocatoria Juan de la Cierva-formación, incluida en el Programa Estatal para Desarrollar, Atraer y Retener Talento, del Plan Estatal de Investigación Científica, Técnica y de Innovación para el período 2021-2023, en el marco del Plan de Recuperación, Transformación y Resiliencia. Financiado por la Unión Europea -NextGenerationEU.




