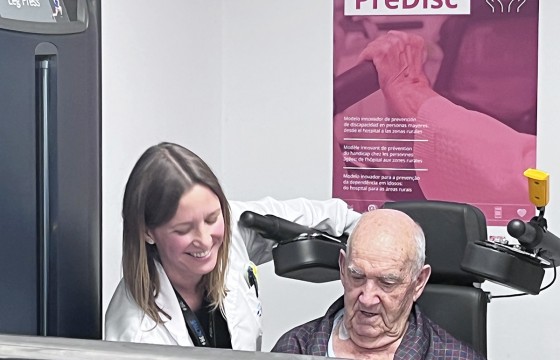
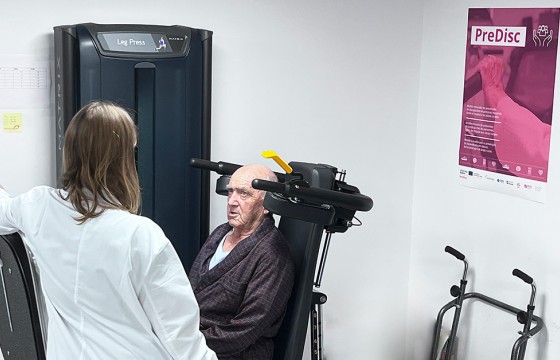
- Navarrabiomed, which leads this transnational cooperation project, presents the model applied in Navarra for the care of elderly hospitalised patients at the launch meeting for PreDisc.
The partners of the PreDisc project came together on Friday 15th March, at the Navarrabiomed biomedical research centre, to launch this transnational cooperation initiative between professionals from France, Portugal, Andorra and Spain, which is cofinanced with FEDER funds via the Sudoe program. The aim thereof is to prevent disability in elderly patients by promoting regular physical activity during and after periods of hospitalisation.
The government of Navarra’s regional health minister, Fernando Domínguez, chaired the launch event for PreDisc, where he highlighted “the importance of implementing a personalised training program during hospitalisation and continuing it after discharge, with follow-up by Primary Care”. The project also includes a multicentre clinical trial to generate scientific evidence and promote the integration of such programs into standard clinical practice.
“Our society is undergoing changes, with more and more elderly people, with more chronic conditions and a greater likelihood of being admitted to hospital, therefore health systems, especially publicly funded ones, must adopt new practices to adapt to this new reality”, explained Domínguez. Similarly, he indicated that “the solutions we can provide to the challenge of ageing are varied, but all point towards innovation as a working goal. Innovation in all its meanings: methodological, social, technological, etc. The PreDisc project is a good example of that”.
Furthermore, as the Minister of Health explained, “international cooperation, collaboration between research, hospital healthcare and Primary Care, the technological commitment, as well as the social and territorial orientation of the project have to generate an innovative model. of disability prevention in the elderly based on the individualised prescription and coordinated monitoring of a physical training program for them.”
Together with representatives of the project’s partners, the head of Navarrabiomed, Maite Mendioroz Iriarte, the Managing Director of the Servicio Navarro de Salud-Osasunbidea, Alfredo Martinez Larrea, and the Managing Director of the Hospital Universitario de Navarra, Estrella Petrina Jáuregui, were also present.
The care model for hospitalised elderly patients in Navarra
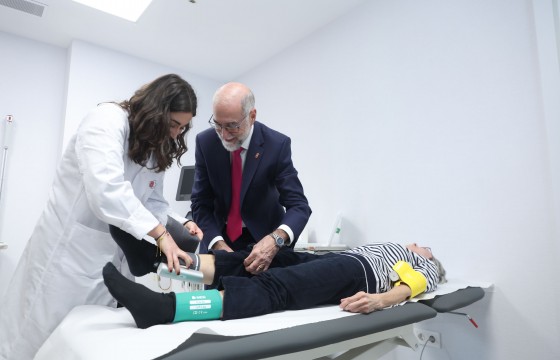
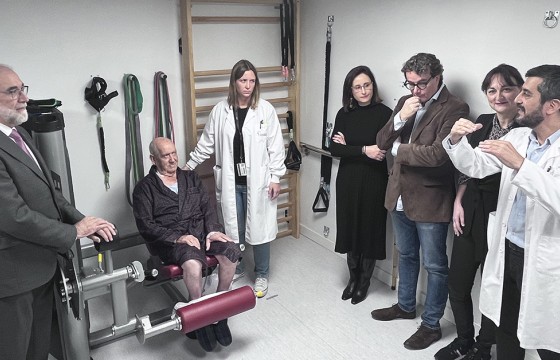
After the institutional event, the attendees moved to the gym located in the HUN Geriatrics department, to learn about the care model for hospitalised elderly patients in Navarra and the impact that physical activity has during their stay in the hospital.
The prescription of personalised physical activity during the hospitalisation period is being progressively integrated into the hospital system in order to prevent the functional deterioration associated with hospitalisation, although it has not yet been fully integrated into standard clinical practice. “The aim is to prioritise functional recovery, thanks to an individualised training program that allows the hospital stay to be shortened and avoids the disability that may arise during hospitalisation. This new care model allows a more comprehensive approach to elderly patients and moves us away from a model more focused on each patient's illness,” explains Nicolás Martínez Velilla, head of the Geriatrics and Active Ageing Unit (INGEA) of Navarrabiomed, head of the Geriatrics Department at HUN and principal investigator of the PreDisc project.
It must be remembered that the demographic situation of the countries in the SUDOE region (France, Spain, Andorra and Portugal), as is also the case for the rest of Europe, is characterized by a progressive ageing of the population, with a life expectancy of around 83–84 years. This phenomenon poses challenges in terms of healthcare and long-term care. To address these challenges, comprehensive strategies that promote active ageing, prevent disability, guarantee universal accessibility and adapt services to the needs of older people are needed. In this sense, geriatric care during the hospitalisation period is crucial in the life trajectory of these patients.
In 2012, a prospective observational study was carried out in patients over 75 years of age admitted to the Geriatric Department of Navarra to evaluate the impact of bed rest on their functionality. It was found that 48.4% of patients suffered functional deterioration upon admission, with this persisting in 38.7% upon discharge. The average time spent in bed was 19.7 hours per day, and 58.1% were bedridden for an average of 20 hours per day. The length of time spent in bed was associated with functional deterioration and mortality, thus suggesting the need for interventions to improve patient care.
A randomized clinical trial conducted in 2019, also at HUN, investigated the impact of an individualised exercise intervention in very elderly people during acute hospitalisation. A total of 370 patients were randomly assigned to an exercise group or a control group. The main findings showed that the exercise group experienced significant improvements in functional capacity, cognitive status, mood, and quality of life compared to the control group. Furthermore, no adverse effects related to the exercise intervention were observed, thereby suggesting that it is safe and effective in reversing the functional decline related to acute hospitalisation in very elderly patients.
To facilitate the integration of physical exercise during the hospitalisation period into standard clinical practice, it is necessary to generate greater scientific evidence through a multicentre clinical trial that will be carried out within the framework of the PreDisc project. In this case, the randomised, multicentre trial will be international and will include the participation of HUN, CHU Toulouse, Hospital Distrital da Figueira da Foz and Hospital Nuestra Señora de Meritxell in Andorra. Its objective is to generate greater scientific evidence and to be able to integrate personalised physical activity programs into standard clinical practice at the hospital level in the future.
From the hospital to rural areas
In addition, to prevent longer-term disability and improve the quality of life of older people in their daily lives, the PreDisc project proposes to continue this physical exercise program started in the hospital after discharge, by way of follow-up in Primary Care and the development of an innovative eHealth tool.
And beyond patient care in the healthcare field, PreDisc also focuses its scope of action on the promotion of active ageing, especially in rural areas, through the development of technological tools, the training of healthcare personnel, carrying out home visits and events in rural communities, which should reduce unnecessary hospital admissions.
PreDisc has a total budget of 1,288,550 euros, 75% of which is co-financed by the Interreg Sudoe Program, which supports regional development in south-western Europe by collaborating in the promotion of transnational projects through the European Regional Development Fund (FEDER). This program promotes cooperation to resolve problems common to the regions of southwestern Europe.
The project consortium is led by the Navarrabiomed biomedical research centre - Fundación Miguel Servet and includes the participation of the Navarra Health Research Institute (IdiSNA), Primary Care of the Servicio Navarro de Salud – Osasunbidea (SNS-O), the University of Deusto (Bizkaia, Euskadi), Center Hospitalier Universitaire de Toulouse Pôle Gériatrie (Occitania), Hospital Distrital da Figueira da Foz, EPE (Coimbra, Central Region) and Servei Andorrà d'Atenció Sanitària (Andorra), supported in turn by nine public and private organisations in southwestern Europe that participate as associate partners.